Jeff Fernandes
From L to R: Tony Hollenberg, MD, Physician-in-Chief, Chris Andry, MPhil,PhD, Chief of Pathology & Laboratory Medicine, Anh Tran, MS, BSN, Clinical Director, Johanna Chesley, Senior Director, Center for Clinical Research Advancement, Archana Asundi, MD, Medical Director, Alastair Bell, MD, President & CEO, Megan Bair-Merritt MD,MSCE, Chief Scientific Officer, Nancy Hanright, Senior Director Real Estate and Capital Planning
The Boston community will have the ability to participate in Phase 1 clinical trials, providing access to cutting-edge, potentially lifesaving treatments and therapies.
Clinical trials have disproportionately excluded participants of color. According to a 2022 study of 32,000 individuals who participated in new drug trials in the U.S., 75% were white, and only 11% were Hispanic and 7% were Black — far below overall population representation rates. This has two important consequences for healthcare. One, as a result, therapies are being developed without people of color in mind, despite the fact that the same communities are disproportionately affected by chronic diseases that are of high investigative priority in drug research and development. Two, people who are already often underserved in healthcare are not having equitable access to potentially life-saving new therapies.
As part of its overall focus on equity and innovation, Boston Medical Center (BMC) is working to change this research landscape. A key initiative is the launch of its new Clinical Research Unit (CRU) this month, the first of its kind for an essential hospital in Boston.
“It’s a strong demonstration of BMC’s commitment to innovative, inclusive care,” says Ryan Schroeder, BMC’s director of business operations for the Clinical Research Unit. “[The CRU] effectively transfers power to our communities to engage in the clinical trial process.”
The new unit will not only enable researchers to conduct trials on transformative therapies, it will increase access to these therapies for its patient population, nearly 70% of whom are people of color.
A one-stop shop for clinical research creates efficiencies
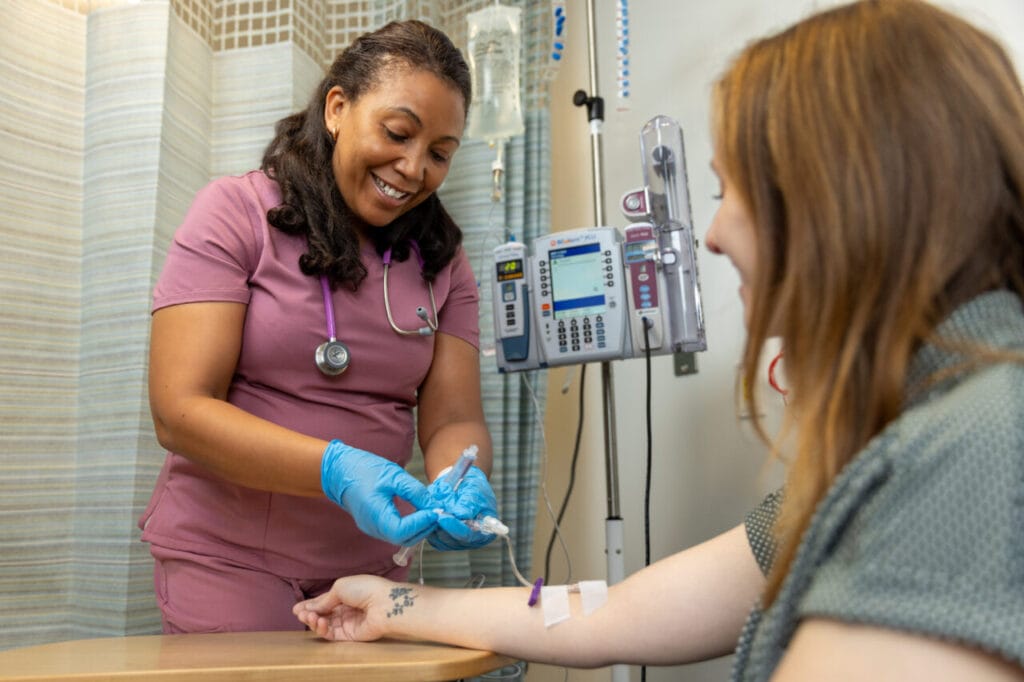
Located on BMC’s main campus, the new unit boasts 3,000 square feet of state-of-the-art-in-hospital clinical research space. The unit houses traditional exam rooms as well as overnight infusion and observation spaces, plus spaces for medication administration and phlebotomy and lab processing — all under one roof. The core benefits of this layout are operational efficiencies and strengthening patient-provider relationships.
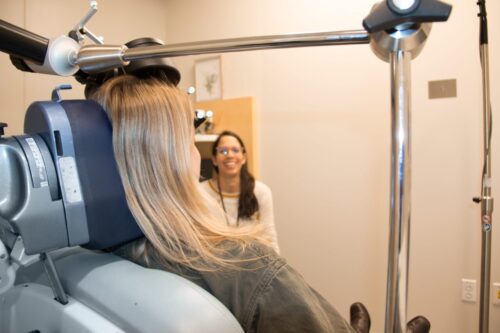
The broad umbrella of resources and services housed under the CRU positions the unit to be a one-stop-shop for clinical trial research activity.
“What is typically seen a clinical trial setting is that a patient will go to one location where a specimen is collected, the specimen then goes to a separate lab space for initial processing, the lab then ships it out to another location for study specific tests and storage,” Archana Asundi, MD, medical director of the CRU explains. “The seamless integration into one space effectively streamlines the research process and increases efficiency across all research activity.”
The design of space lends it self to efficient research models and operational harmonies within the research lifecycles.
Moreover, The CRU’s in-hospital location, specifically its close proximity to the Emergency Department bolsters its capacity for observation and enhances patient safety. (In addition, the unit has both adult and pediatric code carts.) Before the CRU, BMC researchers were limited in their ability to conduct certain trials due to safety constraints. With the unit, they can now take on more expansive studies with higher clinical risk and/or that require overnight stays.
New Clinical Research Unit efficiencies benefit patients
This one-stop-shop feature also enables patients to take part in all phases of the clinical trial in one medical home. Whereas often, clinical trial participants can expect to visit multiple sites from start to finish, at the CRU, they ease their burden and, ideally, build trust with their team.
“At our new CRU, we manage all interactions with our patients through the entire process, which is not only beneficial on the operational side, but it also allows us to connect more personally with our patients,” Asundi says.
“It’s a strong demonstration of BMC’s commitment to innovative, inclusive care … [The CRU] effectively transfers power to our communities to engage in the clinical trial process.”
Ryan Schroeder, BMC’s director of business operations for the Clinical Research Unit.
The interpersonal aspect of patient provider relationships is of particular relevance to BMC patients who have valid distrust of medical systems. A 2020 NIH study revealed that historic lack of trust between medical institutions and underserved communities is often framed as something that needs to be changed on the patient side, rather than what can and should be changed on the organizational side. By opening the unit, BMC seeks to look within to help address this historic distrust with its provider teams.
“The provider teams in the unit reflect the community that we will serve.” says Anh Tran, MS, BSN, clinical director of the CRU. “And when patients can converse in their primary language with our providers, it helps to build a more trustworthy environment.”
The positive effects of a diverse research staff were also found in a 2023 BMC-led research study that analyzed patient consent rates in ophthalmological clinical trials. The study found that when a provider was of the same race or ethnicity as the patient, patient consent rose by 25%, effectively increasing racial diversity within the clinical trial enrollment — a core goal of the CRU.
New research pathways

This new space has the potential to expand research opportunities by fostering new models of collaboration that may not have otherwise been nurtured. Because the unit itself is not tied to any department, it is primed to facilitate interdisciplinary and convergent models of collaboration that were not possible before, according to Asundi.
“It will host a wealth of knowledge that will bolster the quality of research we conduct,” she says. “the merging of researchers under one physical space, that may not otherwise have met due to physical departmental silos, may lead to new innovations that would not have otherwise come about.”
It’s that wealth of knowledge joining together in research via the new CRU that’s so important, according to Schroeder, who quickly adds, “Which leads to better care for our patients.”